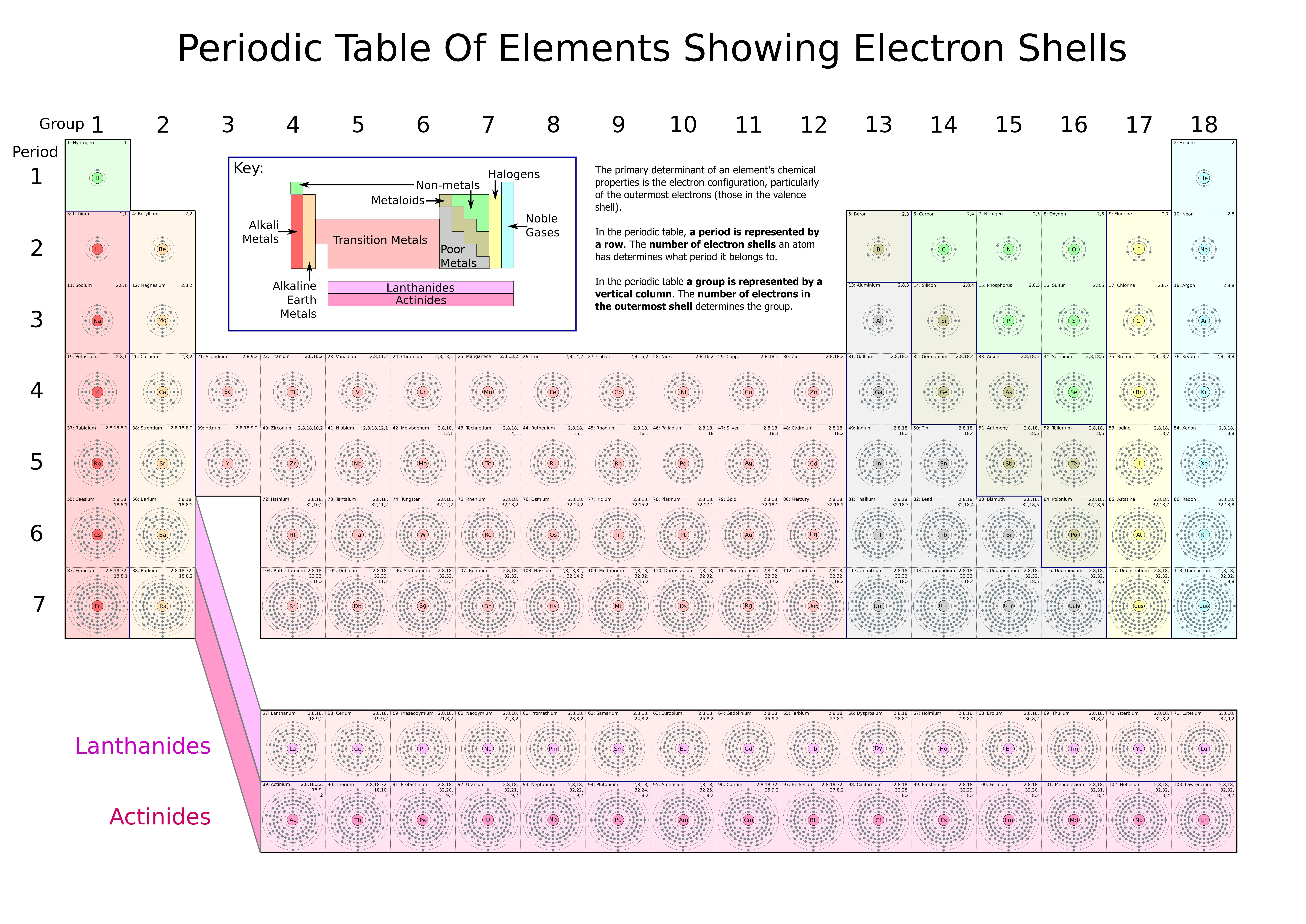
Iron Has How Many Electrons In The Shells . in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. iron atoms have 26 electrons and the shell structure is 2.8.14.2. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. the periodic table, electron shells, and orbitals (article) | khan academy. The ground state electron configuration of ground state gaseous neutral iron is. As a result, iron has eight valence electrons. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,.
from commons.wikimedia.org
the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. the periodic table, electron shells, and orbitals (article) | khan academy. As a result, iron has eight valence electrons. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electron configuration of ground state gaseous neutral iron is.
FilePeriodic table of elements showing electron shells.png
Iron Has How Many Electrons In The Shells the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. the periodic table, electron shells, and orbitals (article) | khan academy. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons. The ground state electron configuration of ground state gaseous neutral iron is.
From valenceelectrons.com
How to Find the Valence Electrons for Titanium (Ti)? Iron Has How Many Electrons In The Shells the periodic table, electron shells, and orbitals (article) | khan academy. iron atoms have 26 electrons and the shell structure is 2.8.14.2. in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. the reason being that there are three different subshells in the. Iron Has How Many Electrons In The Shells.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Iron Has How Many Electrons In The Shells iron atoms have 26 electrons and the shell structure is 2.8.14.2. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. in order to write the iron electron configuration we first need to know the number of electrons for the. Iron Has How Many Electrons In The Shells.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Iron Has How Many Electrons In The Shells As a result, iron has eight valence electrons. iron atoms have 26 electrons and the shell structure is 2.8.14.2. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. in order to write the iron electron configuration we first need. Iron Has How Many Electrons In The Shells.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Iron Has How Many Electrons In The Shells As a result, iron has eight valence electrons. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. . Iron Has How Many Electrons In The Shells.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor Iron Has How Many Electrons In The Shells the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. iron atoms have 26 electrons and the shell. Iron Has How Many Electrons In The Shells.
From www.gauthmath.com
How is this model useful? It shows how electrons are distributed in the Iron Has How Many Electrons In The Shells in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. iron atoms have 26 electrons and the. Iron Has How Many Electrons In The Shells.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table Iron Has How Many Electrons In The Shells The ground state electron configuration of ground state gaseous neutral iron is. iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the periodic table, electron. Iron Has How Many Electrons In The Shells.
From dxoawusbn.blob.core.windows.net
Iron Electron Configuration Long Form at Dennis Amaral blog Iron Has How Many Electrons In The Shells As a result, iron has eight valence electrons. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. . Iron Has How Many Electrons In The Shells.
From www.slideserve.com
PPT Metals. Metalloids. Nonmetals. PowerPoint Presentation, free Iron Has How Many Electrons In The Shells The ground state electron configuration of ground state gaseous neutral iron is. iron atoms have 26 electrons and the shell structure is 2.8.14.2. As a result, iron has eight valence electrons. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. in layman’s terms, the. Iron Has How Many Electrons In The Shells.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Has How Many Electrons In The Shells the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of. As a result, iron has eight valence electrons. in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. the periodic table, electron. Iron Has How Many Electrons In The Shells.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Iron Has How Many Electrons In The Shells the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. the periodic table, electron shells, and orbitals (article) | khan academy. the general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for. Iron Has How Many Electrons In The Shells.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Has How Many Electrons In The Shells the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electron configuration of ground state gaseous neutral iron is. the general rule stating the number of. Iron Has How Many Electrons In The Shells.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Has How Many Electrons In The Shells the periodic table, electron shells, and orbitals (article) | khan academy. in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. As a result, iron has eight valence electrons. the reason being that there are three different subshells in the third shell (called s,. Iron Has How Many Electrons In The Shells.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Has How Many Electrons In The Shells The ground state electron configuration of ground state gaseous neutral iron is. the periodic table, electron shells, and orbitals (article) | khan academy. the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. the general rule stating the number of. Iron Has How Many Electrons In The Shells.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Has How Many Electrons In The Shells iron atoms have 26 electrons and the shell structure is 2.8.14.2. the periodic table, electron shells, and orbitals (article) | khan academy. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. The ground state electron configuration of ground state gaseous neutral iron is. . Iron Has How Many Electrons In The Shells.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6667703 Iron Has How Many Electrons In The Shells iron atoms have 26 electrons and the shell structure is 2.8.14.2. the periodic table, electron shells, and orbitals (article) | khan academy. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. in layman’s terms, the number of electrons an element can receive, lose,. Iron Has How Many Electrons In The Shells.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Iron Has How Many Electrons In The Shells in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. in layman’s terms, the number of electrons an element can receive, lose, or share in order to fill its outermost electron shell completely. iron atoms have 26 electrons and the shell structure is 2.8.14.2. As. Iron Has How Many Electrons In The Shells.
From www.vedantu.com
Distribution of Electrons in Different Orbits/Shells Learn Important Iron Has How Many Electrons In The Shells the reason being that there are three different subshells in the third shell (called s, p and d orbitals) which can contain 2, 6 and 10 electrons,. The ground state electron configuration of ground state gaseous neutral iron is. iron atoms have 26 electrons and the shell structure is 2.8.14.2. the periodic table, electron shells, and orbitals. Iron Has How Many Electrons In The Shells.